Describe the Arrangement of Particles in a Liquid
They get the vapor title when they are in a gaseous phase. Explain the different diffusion speeds through substances in solid liquid and gas.

Arrangement Of Particles In Phases Of Matter Comparison Expii
ASTM method D4318-10e1At and above the liquid limit w L clay particles are effectively suspended in water.

. Solid are tightly packed usually in a regular pattern. They are almost as close together as a solid but they can slide past each other. An early precursor to the idea of bonded combinations of atoms was the theory of combination via chemical affinityFor example in 1718 building on Boyles conception of combinations of clusters the French chemist Étienne François Geoffroy developed theories of chemical affinity to explain combinations of particles reasoning that a certain alchemical force draws certain.
But in a string-net liquid. In a string-net liquid atoms have apparently unstable arrangement like a liquid but are still consistent in overall pattern like a solid. Solid are tightly packed usually in a regular pattern.
Can both liquid water and steam exist at 100qC. Like you and a guy with a cold on an elevator. Gas vibrate and move freely at high speeds.
You will probably hear the term water vapor which means water in a gas state. Gas are well separated with no regular arrangement. Liquid vibrate move about and slide past each other.
Chemical composition temperature and pressure affect such arrangements and motions of atoms as well as the ways in which they interact. Additionally in vitro FPM is considered more capable of entering the cell than larger particles Gratton et al 2008 such as the PM10 ie the particulate matter with the diameter of 110 μm. The word vapor is used to describe gases that are usually liquids at room temperature.
What strikes your mind when you hear the word rock. But in vivo particles are difficult to directly contact with cells due to the barrier effects from sticky body fluids and gel-like ECM proteins Fernandes et al 2009. The mass of the sugar is consereved but is just dissolved in the water and therefore spreads out in the liquid The sugar molecules cannot go away but they can disperse in the water.
Label the graph with liquid. Describe the kinetic molecular theory of matter. Basics - PhET Interactive Simulations.
Key Takeaways Key Points. Show the animation Liquid in a Bottle. Tell students that an atom is the smallest building.
A straight wall could have been obtained only by a very particular arrangement. Box 33 a water content where the soil or clay mass flows under the influence of gravity cf. Under a given set of conditions the state and some properties eg density elasticity viscosity are the.
All particles have energy and the energy varies depending on the temperature the sample of matter is in which determines if the substance is a solid liquid or gas. Tell students that everything they can see and touch is called matterExplain that all matter on Earth exists in the form of a solid liquid or gas and that solids liquids and gases are all made of extremely tiny particles called atoms and molecules. The units of concentration we just discussed are used to describe the degree to which a solute is soluble in a solvent.
William Bleam in Soil and Environmental Chemistry Second Edition 2017. Show an animation and discuss the motion and arrangement of the particles of a liquid. The arrangement and motion of atoms vary in characteristic ways depending on the substance and its current state eg solid liquid.
Label the graph with gas. Particles used in this research were also taken from the separator on a gas well in Queensland. In the process we were able to.
Until recently tiny particles that are used to make useful materials have been treated the same way the apprentice treated his stones. The particle fraction analysis is presented in Table 2A microscopic image of the particle sample as well as its particle size distribution is provided in Fig. Formation of Sedimentary Rocks.
Investigate and explain. Gas vibrate and move freely at high speeds. However they usually have.
They will still be sugar molecules just not attached to any other molecules of sugar. What must be changed temperature or heat energy during condensation. Using this principle these authors describe a two-step size sorting process in order to obtain significant amounts of nanometric monosized particles with diameters between typically 6 and 13 nm.
Liquid are close together with no regular arrangement. 1The particle sizes were measured using a Malvern Mastersizer 3000 Particle Size Analyzer. Explain that the particles of a liquid are attracted much more than the particles of a gas and that they are much closer together.
Describe the arrangement and movement of particles in the liquid state. Liquid vibrate move about and slide past each other. Igneous rocks are sometimes considered primary rocks because they crystallize from a liquid.
As the surface of the latter is not modified by the size sorting process usual procedures are used to disperse them in several aqueous or oil-based media. Is that rock music Well not anymore as DrBinocs is here to explain different types of rock. Explain why a balloon would get bigger as it gets hotter.
Numerous models and theories describe the particles as perfectly regular in this case as spheres. This is actually the basis for the cells in our bodies. Explain why a balloon would get bigger as it gains altitude.
Liquid are close together with no regular arrangement. Compounds such as carbon dioxide CO 2 are usually gases at room. The lipids oily fatty acids form.
How would you describe the change in the arrangement of particles as heat energy and temperature increase. Solid particles have the least amount of energy and gas particles have the greatest amount of energy. The common solidliquid extraction techn iques and the relative equipment con ventionally used in the food industry as well as the ma in applications in certa in food sectors such as sugar tea.
When you place a non-polar molecule in a polar solvent like oil in water the molecules try to minimize surface contact between them. Gas are well separated with no regular arrangement. Do a demonstration to show that a hammer is a hard solid.
When in a normal solid state the atoms of matter align themselves in a grid pattern so that the spin of any electron is the opposite of the spin of all electrons touching it. Using liquid-phase scanning transmission electron microscopy LP-STEM we investigated the interactions that govern the self-assembly of particles in liquid. Good examples of these types of liquids include water H 2 O and mercury Hg.
Sedimentary rocks are the product of 1 weathering of preexisting rocks 2 transport of the weathering products 3 deposition of the. The temperature of a substance. Label the graph with solid.
Soils with an appreciable clay fraction and virtually all clay minerals have a liquid limit w L cf. In that case sedimentary rocks are derived rocks because they are formed from fragments of pre-existing rocks.
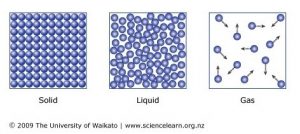
5 10 Describe The Arrangement And Motion Of Particles In Solids Liquids And Gases Tutormyself Chemistry
How Are Particles Arranged In The Three States Of Matter Quora

Arrangement Of Particles In Phases Of Matter Comparison Expii

Draw Diagram Showing The Arrangement Of Particles In Solid Liquid And Gases Brainly In
Comments
Post a Comment